As COVID-19 spreads across the world, falsified medicines for the novel coronavirus are leaking into Africa, where almost 19 percent of medicines are already substandard and where a number of countries are promoting untested treatments for the virus. The global health community is funneling tens of billions of dollars of aid into procuring medical products for countries without full evidence of their safety, efficacy, or quality—let alone their cost effectiveness.
To guard against adverse effects and incorrect prescribing
The pandemic is a brutal and urgent wake-up call that low- and middle-income countries need more effective, efficient regulatory systems. They need to be able to conduct drug and vaccine reviews and expedite registration, prioritize imports, and quickly set up standards for domestic manufacturing. When new drugs hit the market, including new and repurposed products for COVID-19, governments need to monitor their use to guard against adverse effects and incorrect prescribing.

However, regulators in low- and middle-income countries face significant challenges, including inadequate funding, lack of human resources, a dearth of subject matter expertise, and poor regulatory frameworks.
WHO estimates a third of the world's population lacks access to quality-assured medicines
As a result, the bureaucratic process for assessing and approving medicines takes too long and is too scattershot to be effective even for the significant health challenges these countries already face in normal times—let alone during a pandemic or another crisis.
The World Health Organization (WHO) estimates that only 30 percent of its member states have the capacity to effectively and efficiently regulate medical products in their countries, and a third of the world's population lacks access to quality-assured medicines. We need a radically different mindset.
A Bird's-Eye View of Access to Medicines
The global health community must pay much more attention to supporting regulatory capacity in low- and middle-income countries to fill both emergency and ongoing needs. Medicines are not a simple commodity. While operations, logistics, and cost are important, it takes a web of institutions, processes, and people to ensure reliable access to safe, effective, quality-assured medical products. All of the components of a sound regulatory system need time, money, and commitment to put into place and achieve the long-term pay off.
Countries need a decision-making structure that has the authority and oversight to institute sound policies and enforce laws
Take governance, for example. Countries need a decision-making structure that has the authority and oversight to institute sound policies and enforce laws. Following the Ebola outbreak in Sierra Leone in 2015, our organization, Management Sciences for Health, supported the country in helping its new directorate of drugs and medical supplies figure out its structure, duties, and five-year operations plan. Our organization also helped the country pass legislation establishing a public service agency responsible for medicine procurement, warehousing, and distribution. In Swaziland, Management Sciences for Health assisted the ministry of health draft the Medicines and Related Substances Control bill, which provided a legal mandate for establishing the first-ever regulatory body in the country.
Information management systems also facilitate regulatory decision making. As recently as 2016, it took Mozambique an average of four hundred days for medicine importers and distributors to receive market authorization. In 2017, Mozambique implemented an electronic system for medicines regulations, making the process much speedier, more accurate, and more transparent. It is also adopting a web-based system for monitoring medicines safety called PViMS.
A Game-Changing Tool for Systemic Improvement
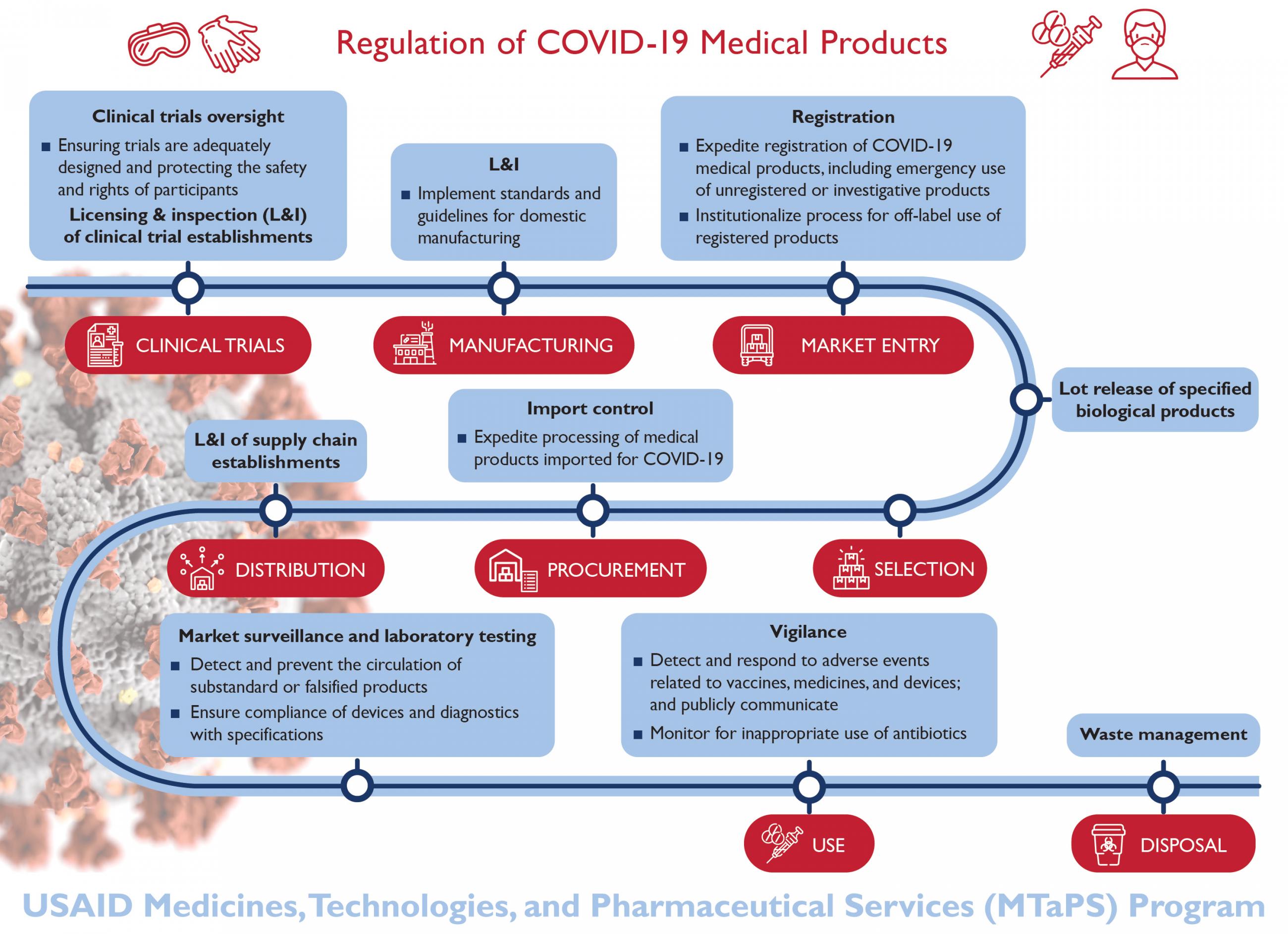
Countries also need a way to assess where they stand and plot a course to improve their regulatory systems. WHO recently finalized the Global Benchmarking Tool, the first internationally agreed-upon way to assess how well national medicines regulatory systems function and to create an institutional development plan.

The tool's indicators cover the myriad areas needed to get the job done: the overall regulatory system, registration, pharmacovigilance, market surveillance and control, establishment licensing and inspections, laboratory testing, clinical trials oversight, and vaccines release. So far, seventy-five countries have completed self-assessments or formal benchmarking with WHO and are ready to move on to the hard work of designing and implementing plans to build their capability. Donors, global health professionals, and governments must gather the political will and momentum to press for real change.
Ensuring people have safe and reliable medical products going forward
As we struggle to meet emergency needs during the pandemic, we have a window of opportunity to overhaul the way we handle the regulation of medical products. Helping countries deploy the global benchmarking tool is the first step toward strengthening regulatory systems. Having stronger systems in place will lead to a faster response, at a smaller scale, than wholesale emergency interventions started from scratch and will make sure that people have safe and reliable medical products going forward.












