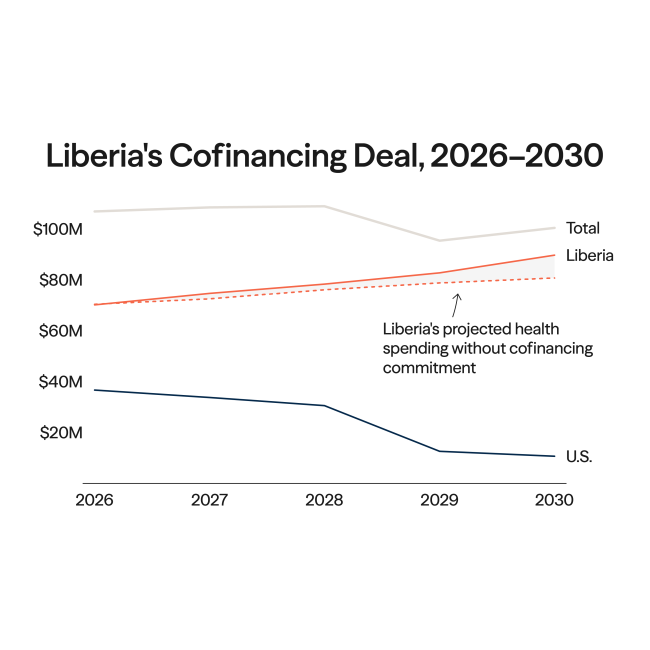
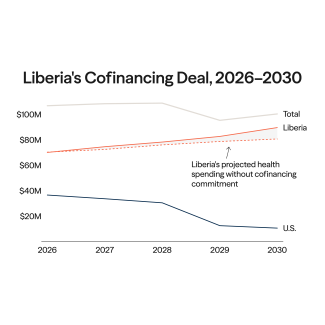
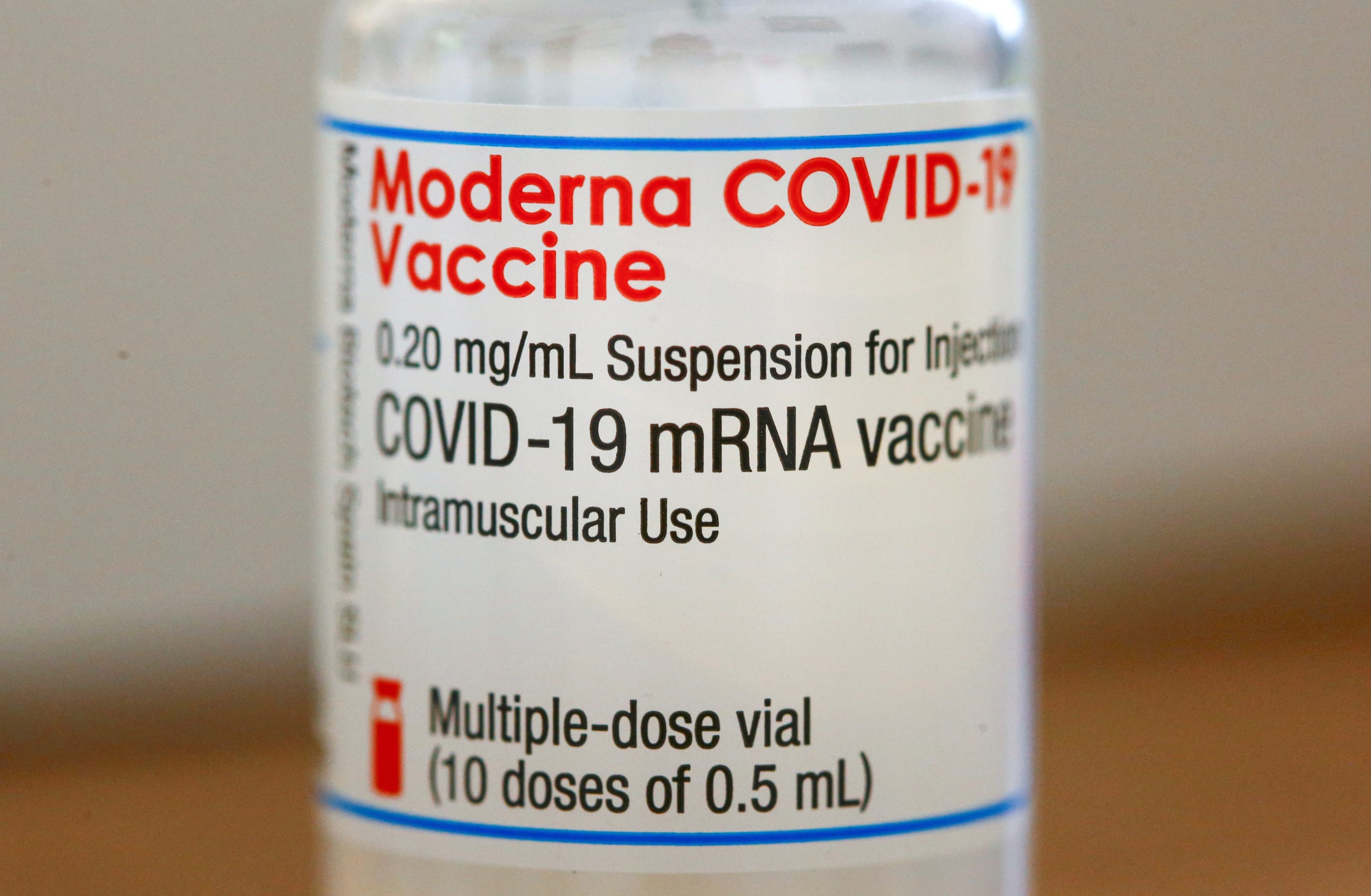
A public discourse, long thought settled by scientists and doctors, has resurfaced around the safety of thimerosal, an ethyl mercury-based preservative used in some vaccines.
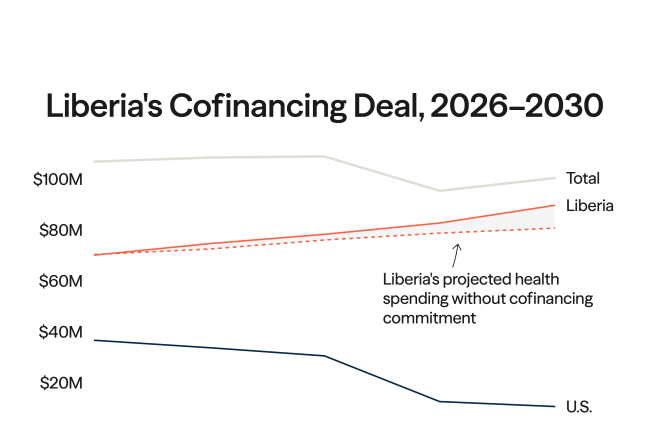
On January 28, 2026, Reuters reported that the Trump administration informed Gavi, the Vaccine Alliance to phase out immunizations containing thimerosal in order to receive U.S. funding.
This ultimatum follows events in late June 2025, when the Advisory Committee on Immunization Practices (ACIP), a U.S. federal vaccine advisory panel, recommended that Americans stop taking flu vaccines containing this preservative after some ACIP members voiced safety concerns about the compound, despite more than two decades of research consistently showing no evidence of harm associated with its use.
The practical impact is expected to be minimal in the United States because thimerosal is only used in multidose vials (MDVs) of influenza vaccines. Further, 94% of domestic vaccine supply for the 2024–25 flu season was thimerosal free or thimerosal reduced. International implications, however, could be significant.
This U.S. recommendation and the subsequent discourse on its perceived effects could sow doubt among global partners and dangerously undermine public confidence in the safety of thimerosal and multidose vaccines, which are used widely in low- and middle-income countries (LMICs) because of their cost-effectiveness. ACIP's approach to thimerosal could foretell the committee's plans and the global ramifications for other vaccine ingredients such as adjuvants, a primary topic for discussion [PDF] as the influential panel reconvenes this week.
What Is Thimerosal and Why Is It Used
Since the 1930s, vaccines have included thimerosal to increase the safety of storing MDVs because the preservative prevents bacterial and fungal contamination. Clinics use MDVs so they can immunize more than one person using the same small bottle of vaccine. A fresh needle and syringe are used each time, but merely handling a vial a second time elevates the chances of germs on a person's hands getting inside the vial. Tragically, in the era before preservatives were used, contaminated MDVs caused deadly bacterial infections.
The ingredient has no scientific evidence of toxicity and no evidence of harm from the low concentration used in vaccines
The addition of thimerosal solved this safety problem, and the ingredient has no scientific evidence of toxicity and no evidence of harm from the low concentration used in vaccines.
In settings where packaging and utilizing vaccines in single-use vials are impractical, as is often the case in many LMICs, multidose presentations allow a single vial to deliver 5 to 20 doses, dramatically cutting costs and easing cold chain logistics. Every day at immunization programs across the globe, a health worker opens an MDV, withdraws what's needed for a single child, and then gives the vaccination.
This process is repeated for all the children requiring that vaccine during the session. In the evening, the health worker can store a semi-depleted vial in the fridge. The following day, if enough remains in the vial, it can be used again to give doses to new children. Provided that the vial stays cold, health workers can safely use this MDV for nearly a month even if only one or two children were vaccinated per day.
An Uneasy Switch from Multidose to Single-Dose Vials
Eliminating thimerosal as a preservative from MDVs without having a suitable replacement readily available would have sweeping implications for immunization in LMICs. With careful planning and a steady focus on innovation, this change could be managed to minimize negative consequences and maximize opportunities for strengthening both supply and demand in these areas. However, if done arbitrarily and abruptly, systems will experience avoidable and predictable roadblocks.
Cold chain capacity. Multidose vials are extremely efficient for storage and distribution. Replacing all 10-dose vials with single-dose packaging would increase storage needs three- to fourfold. Many health facilities in LMICs already face cold chain constraints and a sudden surge in demand could be unsustainable.
Production and cost. Switching to single-dose glass vials means significantly more material to manufacture, inspect, and ship. This inefficiency translates into higher production costs and reduced throughput. The production of daily doses could decline by about 95% unless stakeholders make additional investments in vial filling equipment. For the poorest countries, which pay less than $0.20 per dose of many vaccines through GAVI/UNICEF (United Nations Children's Fund) procurement, absorbing these cost increases would be impossible, especially with GAVI funding under strain.
Speed of vaccine administration. Health staff would face a marginal increase in workload because more vials must be opened and administered to vaccinate the same number of children.

Can't Multidose Vials Use Another Preservative?
Many studies assure thimerosal's safety because the ingredient has featured in billions of vaccine doses with an extremely low rate of adverse reactions. However, given political and pragmatic considerations, there should be interest in alternative preservatives that could replace thimerosal in MDVs. The leading candidate is 2phenoxyethanol (2PE), which is commonly used as a preservative in cosmetics, eye drops, and oral antiseptics. 2PE is also already used in some licensed vaccines, such as the inactivated polio vaccine (IPV) and the pneumococcal conjugate vaccine (PCV13), demonstrating its viability as an alternative. In 2017, Pfizer introduced a four-dose multidose vial of PCV13 using 2PE as the preservative, which allowed LMICs to have a PCV vaccine with a smaller cold chain footprint and lower packaging cost per dose.
But switching to 2PE for all vaccines would be a significant undertaking that would require the careful alignment of demand and supply as well as tight coordination across actors. Replacing thimerosal with 2PE would involve reformulating vaccines and conducting additional testing. Manufacturers would also need to perform preservative efficacy studies to demonstrate that 2PE effectively eliminates bacteria and fungi within defined time frames. Further, regulatory authorities will require data proving that vaccine safety and efficacy remain unchanged; in some cases, small bridging studies could be necessary to compare the immune response of vaccines preserved with 2PE versus thimerosal.
Production facilities would also need to run validation batches to ensure that quality remains unaffected, which demands both time and resources. Although producing industrial-scale availability of 2PE is unlikely to pose a bottleneck, manufacturers must verify sourcing from high-quality suppliers and confirm that supply chains are reliable and sufficiently diversified.
Despite these considerations, experiences with vaccines such as PCV13 and IPV demonstrate that reformulation with 2PE is technically feasible. The greatest challenge lies less in the science or manufacturing process, and more in managing coordination across multiple actors to ensure a smooth and synchronized transition.
Further, this approach may present an alternate avenue for preserving multidose vials, but does not address the issue of growing misinformation surrounding preservative safety.
New Packaging Technology
Another alternative lies in packaging. Vaccinologists, toxicologists, and biomedical engineers could create innovative packaging formats that enable single-dose vaccine delivery while mitigating the drawbacks of traditional vials. Blow-fill-seal (BFS) [PDF] is an automated aseptic process by which melted medical-grade plastic is blown into a mold, filled with vaccine, and sealed in one step. The result is a plastic vial with a twist-off top, similar to a glass ampoule. BFS is cheaper to fill and requires less cold chain volume than glass vials. Single-dose BFS ampoules need no preservative, eliminating thimerosal.
To offset higher packaging and transport volume, manufacturers use strips on which multiple single-dose ampoules are attached. Health workers can then snap off one ampoule at a time from these multi-monodose strips. The BFS method has challenges. Brief heat exposure during the filling process initially raised concerns of the degradation of heat-sensitive antigens. However, studies show no loss of potency in vaccines such as those for rotavirus, live attenuated influenza, respiratory syncytial virus, and pneumococcal conjugate. Another issue is that plastic is slightly permeable to gas, meaning some BFS vials could require a foil wrapper or pouch to prevent oxygen or moisture ingress.
The Coalition for Epidemic Preparedness Innovations in partnership with Institut Pasteur de Dakar has funded an innovative packaging technology that fills vaccines into sterile plastic pouches (such as IV bags) each capable of holding around 200 doses. These pouches are lighter and more compact then conventional glass vials and can significantly reduce cold chain costs and storage needs. Vaccines can be quickly and easily administered from the pouches, making them well suited for rapid, large-scale immunization during outbreaks and pandemics. Needle-free vaccine patches are another delivery innovation that could change the dynamics of glass vials and hence of MDV and preservatives. These patches, often about the size of a postage stamp, have an array of microscopic needles on one side that, when pressed onto the skin, painlessly penetrate the outer skin layer and deliver the vaccine either by dissolving or by creating channels for the antigen to diffuse. After a short application, the patch is removed and disposed of. Although needle-free patches were authorized for some COVID vaccines, they are not a mainstream technology and could be more expensive to produce per dose than filling multidose vials.
What Does the Future Hold?
Although all existing evidence points to thimerosal being safe, its stigma and perceived risk underscore the importance of exploring alternative options for a world without thimerosal in MDVs.
This search should be pursued systematically, balancing cost, cold chain capacity, and administration logistics, with safety and efficacy as the top priority. Alternatives such as 2PE could preserve the MDV system but will require substantial regulatory work to gain approvals, and the future offers no guarantee that the same complaints over safety won't emerge.
Over the medium to long term, investing in innovations holds promise. Careful planning, realistic timelines, and intelligent market-shaping efforts such as those that reduced prices for vaccines and cold chain equipment, will be essential to drive affordability and widescale adoption of these innovative delivery formats.
Progress requires close coordination across stakeholders, and stakeholders should expect that more financial resources are needed for any shift away from thimerosal usage. GAVI and UNICEF, as the primary funder and procurer of vaccines for LMICs, will need resourcing to accelerate the transition to 2PE and novel packaging formats. Its procurement signals will carry significant weight in influencing manufacturers' adoption of new preservatives or packaging technologies. Equally important is the involvement of immunization programs to ensure that the transition is practical and sustainable within health systems in LMICs.
It's critical to recognize that reformulation and packaging changes will vary based on antigen compatibility. Not every vaccine can be easily reformulated or repackaged. Prioritization is therefore needed, beginning with vaccines where change is most feasible, cost effective, or urgently required.
EDITOR'S NOTE: This draft was originally published on December 4, 2025.