Ongoing reports on bird flu—infected dairy cattle, infected poultry and egg-laying facilities, tainted raw milk and pet food, and sporadic cases in people through contact with wild birds, poultry, and cattle—recently took a dangerous turn.
Just months into a new surveillance program for highly pathogenic avian influenza (HPAI) in dairy cows, the Animal and Plant Health Inspection Service (APHIS) at the U.S. Department of Agriculture (USDA) confirmed two separate, novel spillover events of H5N1.
On January 31 this year, APHIS identified H5N1 genotype D1.1 in milk silos, which was traced back to multiple farms in Nevada, and uncovered a case of mild conjunctivitis in a dairy worker. Less than two weeks later, on February 13, the same genotype appeared in a milk silo in Arizona. This is the same genotype—variant of a virus classified by its genetic sequence—that recently hospitalized a backyard flock owner in Wyoming and a poultry farm worker in Ohio.
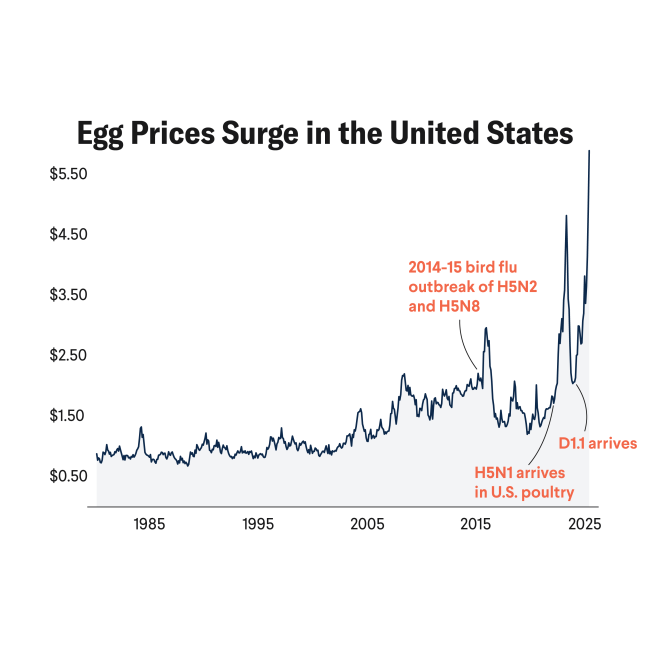
These three avian-to-cow spillover events coupled with 130 infections in poultry flocks in February 2025 raise serious concerns that H5N1 introductions could overwhelm USDA's current containment strategy for dairy cows and the established biocontainment for egg and poultry producing facilities. Concurrently, U.S. government employees engaged in critical response activities have been inexplicably fired. Given the broad and ongoing impacts to the food system (particularly to the egg supply), combined with complicated transmission pathways (see figure), a new strategy is needed urgently.
Mapping H5N1 Spread
Pathways of introduction and transmission, with nodes of risk and surveillance for H5N1

H5N1 Biosecurity for Cows and Poultry: What works?
H5N1 continues to surprise the public health field with spillovers into new species. In early 2024, the original dairy-associated H5N1 genotype—B3.13—emerged, likely triggered by a spillover event from wild birds into cows in late 2023. The event came with major shifts. Avian influenza had entered a new mammal species; cases had the unusual involvement of mammary tissue, affecting milk; and an industry not accustomed to HPAI control strategies—dairy producers—faced a new threat.
One reassurance existed even as the virus spread cow to cow, affecting 17 states and 973 herds: All of these incidents were the result of a single rare spillover event. The virus did not seem to be rapidly evolving in cows. The situation gave hope that restrictions on cow movement, attention to other routes of spread (dairy farm personnel and equipment), and enhanced biosecurity measures—such as improved application and use of personal protective equipment (PPE), decontaminating farm equipment, isolating sick cows, and restricting farm-to-farm movement—could contain the virus from spreading.
This strategy worked well in Colorado, where aggressive, widespread testing and quarantine of dairy facilities allowed the state to return to a negative status. The strategy has not thrived in other states, however, notably California, where prolonged and extensive spread of the original B3.13 genotype has occurred among dairy farms.
California's plight was potentially driven by the close proximity of poultry farms and the timing of the introduction into cattle relative to migration season for waterfowl, although a lack of public epidemiologic data complicates assessment. Despite pilot work by the U.S. Geological Survey that suggested migratory birds use farming spaces where wetlands are not available, more research is needed to understand how wildlife may contribute to transmission among production facilities.
The poultry industry has experience dealing with avian influenza virus outbreaks that includes enhanced biosecurity measures—such as the use of footbaths, restriction of visitors, and exclusion of wildlife from production areas—along with clear guidance for culling, disinfecting, and repopulating facilities. Yet some of these interventions are difficult to implement in dairy cows given the nature of the facilities and the years needed to raise a replacement cow before it can produce milk. By way of contrast, it takes only weeks to months to raise a chicken for meat consumption or egg laying.
H5N1 poses ongoing risks to dairy cow populations that are unlikely to be mitigated by existing strategies for containment
Given the two recent incursions of D1.1, public health experts now know that the potential for H5N1 to spill over into cows is not as slim as once thought. Throughout the 2024–25 winter season, the D1.1 genotype has also infected numerous commercial and backyard poultry facilities, leading to the culling of millions of birds. While D1.1 has been circulating in North American wild birds since September 2024, causing severe disease or death in at least four people, its new spillover into dairy cows demonstrates that the conditions that permitted entry of the B3.13 genotype into these farm animals remain.
The situation suggests that H5N1 poses ongoing risks to dairy cow populations that are unlikely to be mitigated by existing strategies for containment. Even closed herds with dedicated personnel and equipment could be at risk from virus introduction by wild birds or workers and equipment that move between farms. Reengineering dairy production, particularly open free stall designs common facilities in the country, is a monumental task, but one that could be needed to improve biosecurity and limit interactions between wild birds and cows.
H5N1 Threats to Humans: Open Questions
What D1.1's introduction means for human disease remains to be seen. The D1.1 genotype could affect dairy workers differently than B3.13, which has mostly caused mild cases. Although the Nevada dairy worker with D1.1 had mild symptoms, this H5N1 genotype caused severe illness in a Canadian teen, who required life support and was hospitalized for months. The recent human cases in Wyoming and Ohio of exposure with poultry infected with D1.1 have required hospitalization. The H5N1 genotype killed a backyard poultry keeper in Louisiana.
A recent study, released by the Centers for Disease Control and Prevention, tested 150 dairy veterinarians—about half from states with known H5N1 cases in dairy cows—and identified that three people had been infected but showed symptoms between January and September 2024. One veterinarian did not practice in any state known to have ongoing dairy cattle infections during the study period. What role asymptomatic people play in transmission rates is unknown, but this trait could differ between genotypes B3.13 and D1.1. Differences could also exist in their clinical outcomes based on how people were infected.
Many unknowns with this virus are not being adequately addressed through research, surveillance, and timely communication of the risks. Severe cuts in government personnel and federal research funding will make this worse. Although no one can say when or whether this virus will adapt to support efficient human-to-human transmission—or even what mutations would be required—the more times humans and other mammalian species become infected, the greater the risk of further virus adaption.
This scenario is especially worrisome now as regular seasonal influenza case rates and hospitalizations soar near record levels—and for years vaccination rates have hovered at 50% nationally despite wide variations based on geographic location. This environment provides moments where seasonal flu virus circulates in communities alongside H5N1, increasing the risk of an individual or animal catching both viruses at once. Such events can promote genetic reassortment between the viruses, inviting the emergence of a new hybrid potentially capable of spreading from person to person.
Immediate additional steps are needed to increase surveillance, preparedness, and communications about H5N1. They include action to improve surveillance, case detection, and case reporting, as well as to accelerate research and development to include further investment and expansion of research targets. For example, at the time of writing, despite a goal to include participation of all 48 continental states in the National Milk Testing Strategy that identified the new spillover events, three states (Florida, Massachusetts, and North Dakota) are yet to enroll. One—Florida—has a robust dairy industry and allows sales of raw milk for pet use.
A Stronger H5N1 Strategy
Research, testing, and investment are key to a comprehensive bird flu response
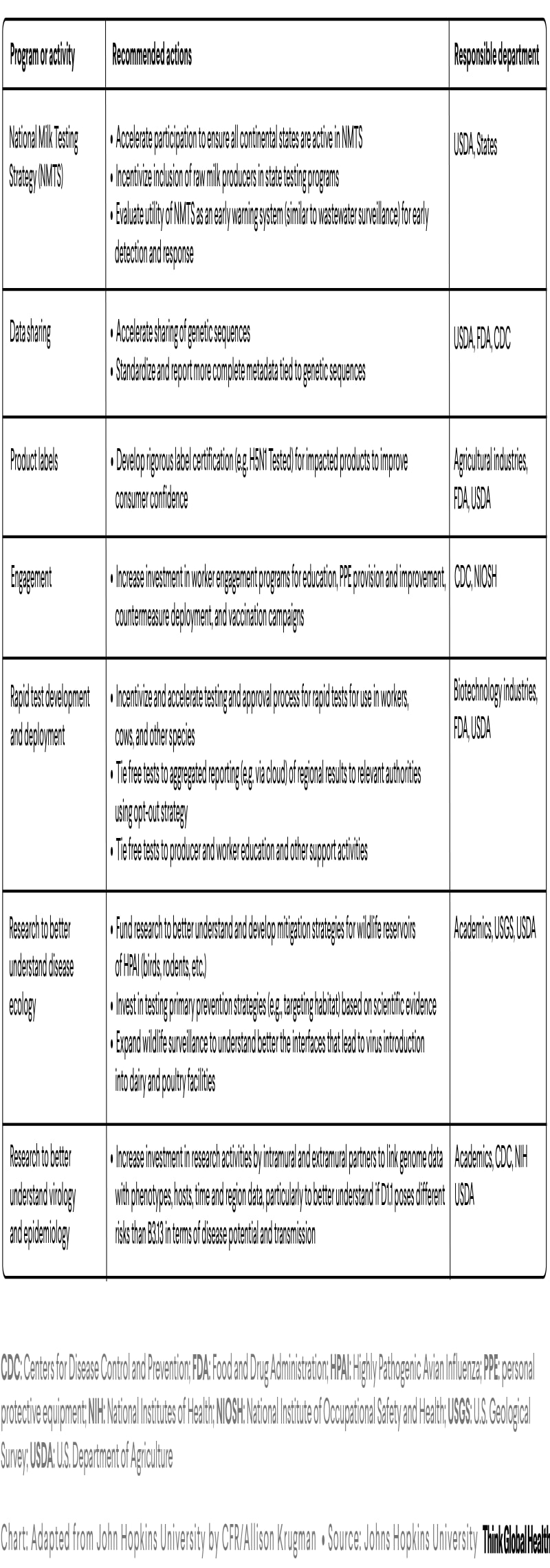
Policies also should be addressed to overcome vaccine hesitancy and systematic barriers for animal vaccination related to trade agreements. The H5 vaccine in the U.S. national stockpile should be effective against the currently circulating D1.1 and B3.13. Officials could allocate these vaccines now to protect at-risk workers and prevent additional human cases. Preventing infection and potential exposure to close contacts (family and pets) would stop the virus from adapting in humans. Officials should take the nontraditional approach of using vaccines to prevent this virus from entering the human population because of the challenges with containing the outbreak in our livestock and wildlife. This tactic contrasts with usual zoonotic approach to stamp out the outbreak in animals before it reaches humans.
The hospitalization of a poultry worker for H5N1 raises additional concerns. The seasonal vaccine is already available for free to all dairy and poultry workers to prevent a coinfection of seasonal with bovine influenza A viruses. Although controversial, rolling out H5N1 vaccine from the stockpile into dairy and poultry workers could provide them with additional protection, plus help generate data to move the vaccine toward federal approval. Finland already vaccinates at-risk populations, and Canada seems poised to follow suit.
Although mass vaccination of cows is a long time off, studies are under way. Vaccinating poultry is a challenge in implementation (related to vaccine costs and trade agreements) and could require constant updating, similar to the human seasonal vaccine. On February 14, the USDA conditionally approved an H5 vaccine from Zoetis for use in poultry to be used in emergency situations or special circumstances.
The time is now to act quickly to ramp up surveillance, invest in research and development, and address policies related to vaccine deployment for poultry and the critical agricultural workforce in the United States.